On the fast track to invasion: intracellular trafficking drives tumor metastasis
Commentary on Giorgio Scita's paper published on Journal of Cell Biology
March 2015
Metastasis, the escape of cancer cells from a primary tumor, and their subsequent dissemination in the body, is invariably coupled with poor patient prognosis. Prevention of metastasis is thus one of the most critical issues in public health management, requiring an in-depth understanding of the regulatory mechanisms. To metastasize, cancer cells have to de-attach from the primary tumor, to both enter and exit the bloodstream, and re-invade into other tissues. During these processes, the cells have to cross a variety of tissue barriers and also to migrate through the dense meshwork of the extracellular matrix (ECM), a fibrillar network that surrounds and connects cells in tissues. To navigate through these terrains, cancer cells have to be able to both adhere to ECM fibres, but also to cleave them to avoid getting stuck. This balancing act is achieved by proteins exposed on the cell surface, in particular by matrix receptors such as integrins and by matrix-lytic enzymes such as metalloproteinases (MMPs). Key proteins of either family are β3 integrins and the matrix metalloproteinase MT1-MMP, both of which are known to drive cancer cell invasion. Accordingly, they are enriched at invadopodia, finger-like protrusions of cancer cells, that are also sites of matrix adhesion and -degradation
The mechanisms by which tumor cells metastasize and the role of endocytic proteins in this process are not well understood. We report that overexpression of the GTPase RAB5A, a master regulator of endocytosis, is predictive of aggressive behavior and metastatic ability in human breast cancers. RAB5A is necessary and sufficient to promote local invasion and distant dissemination of various mammary and nonmammary tumor cell lines, and this prometastatic behavior is associated with increased intratumoral cell motility. Specifically, RAB5A is necessary for the formation of invadosomes, membrane protrusions specialized in extracellular matrix (ECM) degradation. RAB5A promotes RAB4- and RABENOSYN-5-dependent endo/exocytic cycles (EECs) of critical cargos (membrane-type 1 matrix metalloprotease [MT1-MMP] and β3 integrin) required for invadosome formation in response to motogenic stimuli. This trafficking circuitry is necessary for spatially localized hepatocyte growth factor (HGF)/MET signaling that drives invasive, proteolysis-dependent chemotaxis in vitro and for conversion of ductal carcinoma in situ to invasive ductal carcinoma in vivo. Thus, RAB5A/RAB4 EECs promote tumor dissemination by controlling a proteolytic, mesenchymal invasive program.
[PMID 25049275]
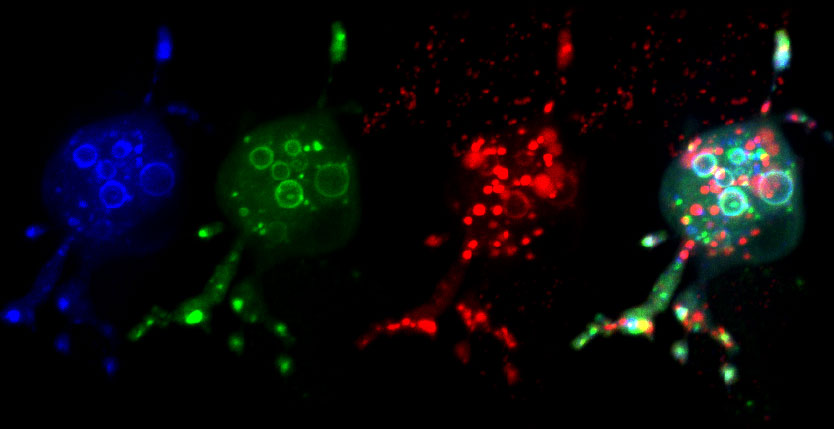
Both β3 integrin and MT1-MMP are anchored to the cell surface by transmembrane domains, which also enables their recycling by internalization, intracellular transport in vesicles and re-exposure at the cell surface. This cycle ensures the dynamic presence of both “grip- and clip-“ functions on the surface of cancer cells during their migration through a changing environment. Intracellular trafficking is thus expected to have a critical impact on cancer cell migration. The paper by Giorgio Scita´s lab now identifies a particularly efficient trafficking pathway, which is characterized by two members of the RabGTPase family, Rab5A and Rab4, and ensures swift recycling of β3 integrin and MT1-MMP during cell invasion.
Analysing the data from 980 breast cancer cases, Frittoli et al. found a striking correlation between expression levels of RAB5A and patient prognosis. In particular, cells from lymph node metastasis showed elevated levels compared to those from primary tumors, indicating a critical role for RAB5A in metastasis, which could also be confirmed in a mouse model. On a cellular level, the Scita group could show that RAB5A is both necessary and sufficient to drive MT1-MMP-dependent cell invasion, which is based on the formation of invadopodia and invadopodia-associated matrix degradation. A closer examination of potential recycling pathways led to the identification of RAB4 and its effector RABENOSYN-5. The latter, being able to bind both RAB4 and RAB5A, apparently acts as the connecting link between both arms of the pathway. Accordingly, the Scita group demonstrated that RAB4 is required for RAB5A-dependent recycling of MT1-MMP and β3 integrin, as well as the formation of invadopodia.
Frittoli et al. thus developed a model in which a RAB5A- and RAB4-dependent circuitry drives fast recycling of MT1-MMP and β3 integrin, thus promoting the formation of matrix-lytic invadopodia. Coming full circle, this model was confirmed by the identification of high levels of RAB4 in invasive breast cancers, and also by reducing the invasiveness of cancer cells, through expression of an inactive mutant of RAB4 in a mouse model of metastasis.
Elegantly combining a wealth of in vivo and in vitro data, this paper identifies two specific RAB GTPases, RAB5A and RAB4, in the intracellular trafficking of key cargo proteins of invadopodia formation and function. The Scita group thus makes a very strong case for paying more attention to RAB proteins in the prediction of clinical outcome, and also for considering these drivers of fast recycling and metastasis formation as potential therapeutical targets.
As most things in nature, however, also RAB5A has a flip side. In primary macrophages, it acts not as a positive, but as a negative regulator of MT1-MMP surface exposure (Wiesner et al., J. Cell Sci., 2013). Discussing and contrasting these findings from our labs has been a pleasure and led to a co-authored commentary article (Linder and Scita, Small GTPases, in press). The Frittoli et al paper is thus a perfect example of not only presenting rock-solid and highly relevant data, but also of sparking discussions, which is the hallmark of great science.